THE DETERMINATION OF ANTIMONY (Sb) IN POLYETHYLENE TEREPHTHALATE (PET)
Polyethylene terephthalate (PET) is a polymer widely used in to make synthetic fibres, films and bottles. PET is the most popular material for food and beverage packaging and is used for carbonated soft drinks, mineral water, edible oils, juices and sauces etc. Antimony trioxide (Sb2O3) is used as a catalyst in the manufacture of PET and has been shown to leach from PET. This leaching is enhanced at higher temperatures and higher acidity. Antimony (Sb) is a regulated contaminant that poses both acute and chronic health issues. The concentration of Sb in PET packaged food and beverages in generally very low (ultra-trace concentrations) with a permissible limit of total Sb in drinking water, established by the US EPA, of 6 μg/L. Sb, however, exists predominately in its (+V) and (+III) oxidation states in water and other drinks sold in PET packaging. These oxidation states exhibit different toxicity behaviour with Sb (+III) being significantly more toxic than Sb (+V). It is important, therefore, to have the capability to determine both total Sb as well as these individual inorganic species at ultra-trace concentrations. |
|
 |
10.055 Millennium Excalibur
|
 |
|
P S Analytical offer instrumental solutions for this application that uses the Millennium Excalibur for total Sb determinations. The limits of detection for Sb is 10ng/L providing ample analytical performance.
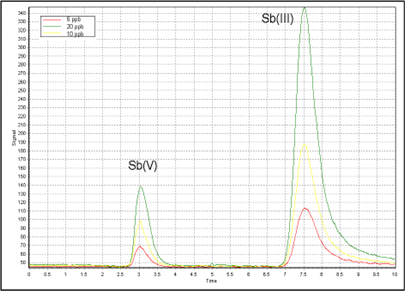
When the Millennium Excalibur is coupled with PSA’s Modular Interface, speciation studies are possible, providing more information on the different Sb forms present. An example chromatogram is provided below.
 |
Application Notes on this topic and others are available. These application notes cover the determination of mercury and the hydride elements from a whole range sample matrices.
To discuss particular requirements and for more detailed information on the above products, please complete the Information Request Form.





